Balanced and Unbalanced Chemical Equation




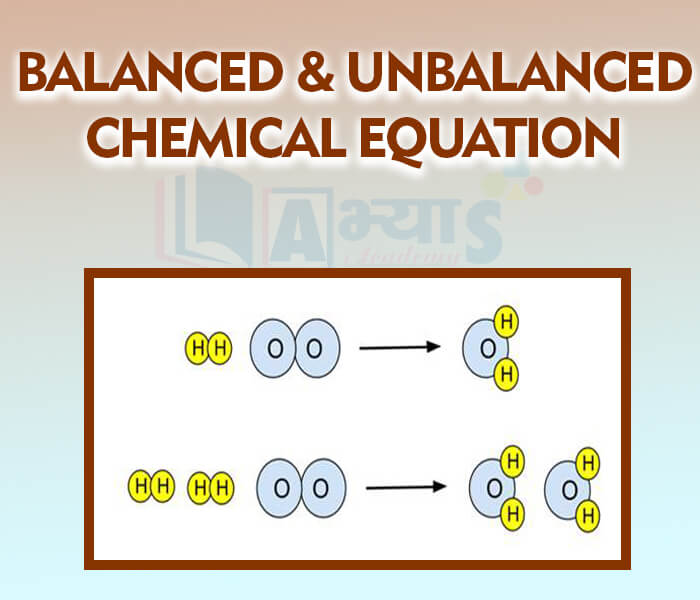
Balanced and Unbalanced Chemical Equation
Balancing an Equation
1) Count the number of atoms of each element on the both sides of equations for example. Magnesium burns in oxygen to give magnesium oxide.
|
Element |
No. of atoms in LHS |
No. of atoms in RHS |
|
Mg |
1 |
1 |
|
O |
2 |
1 |
2. To balance oxygen on both sides, multiply RHS by 2 i.e.
3. Now oxygen atom is balanced but number of magnesium atom is not therefore multiply on the LHS by 2.
Which of the following is a balanced chemical equation of the reaction between carbon monoxide gas and hydrogen gas? | |||
| Right Option : D | |||
| View Explanation | |||
A ____________________ is an equation in which various reactants and products are represented by their respective formulae but no attempt is made to equalize the number of atoms of various elements on both the sides of the equation. | |||
| Right Option : A | |||
| View Explanation | |||
Consider reaction: | |||
| Right Option : B | |||
| View Explanation | |||
Students / Parents Reviews [10]
About Abhyas metholodology the teachers are very nice and hardworking toward students.The Centre Head Mrs Anu Sethi is also a brilliant teacher.Abhyas has taught me how to overcome problems and has always taken my doubts and suppoeted me.

Shreya Shrivastava
8thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.

Eshan Arora
8thMy experience was very good with Abhyas academy. I am studying here from 6th class and I am satisfied by its results in my life. I improved a lot here ahead of school syllabus.

Ayan Ghosh
8thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thI have spent a wonderful time in Abhyas academy. It has made my reasoning more apt, English more stronger and Maths an interesting subject for me. It has given me a habbit of self studying

Yatharthi Sharma
10thIt was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.

Cheshta
10thA marvelous experience with Abhyas. I am glad to share that my ward has achieved more than enough at the Ambala ABHYAS centre. Years have passed on and more and more he has gained. May the centre flourish and develop day by day by the grace of God.

Archit Segal
7thMy experience with Abhyas is very good. I have learnt many things here like vedic maths and reasoning also. Teachers here first take our doubts and then there are assignments to verify our weak points.

Shivam Rana
7thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.
